
Pair of electrons on the oxygen is gonna form a bond between So we're gonna form aīond between the oxygen and this partially positive carbon, so let me say that this lone And that gives the chlorineĪ negative one charge, so this is the chloride anion and we call this a leaving group. So these two electronsĬome off onto the chlorine, so I'll make these this pair. Lone pair of electrons, and let me highlight thoseĮlectrons in magenta.

Pairs of electrons around it, let me go ahead and draw those in. At the same time, these two electrons come off onto the chlorine.

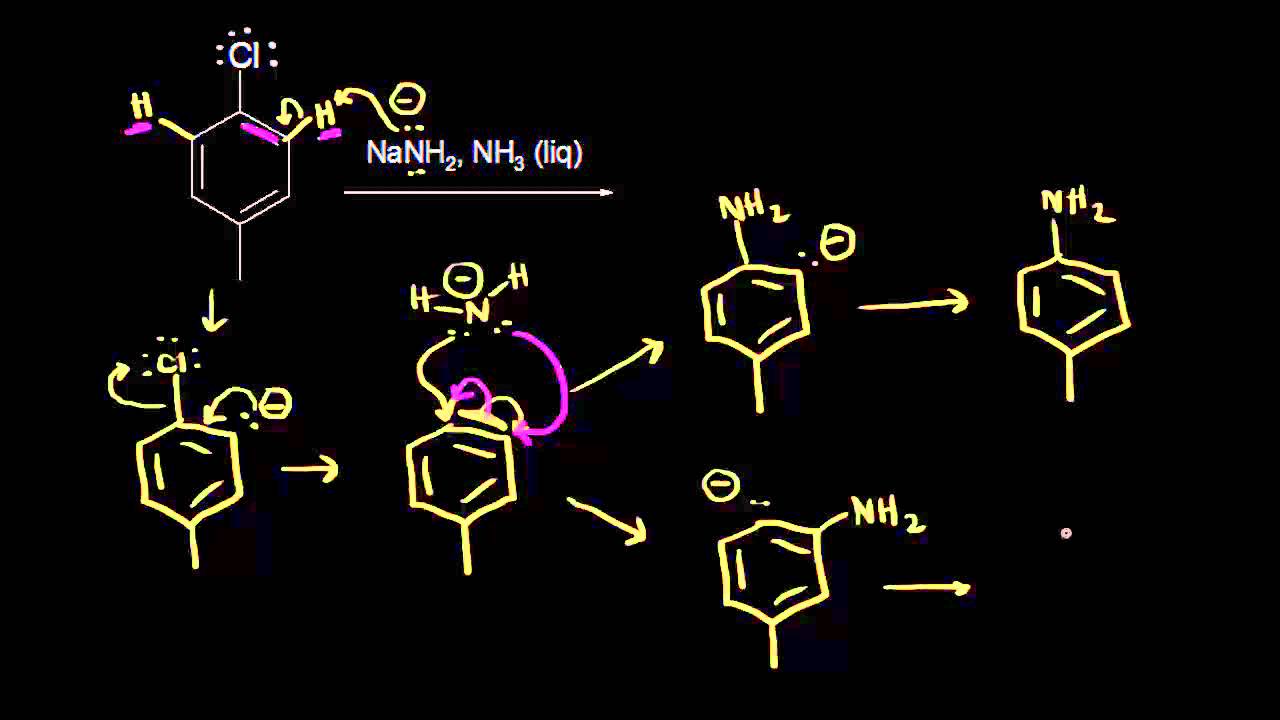
And we say that the nucleophileĪttacks the electrophile, so I could draw a curved arrow showing the movement of two electrons over here to this carbon. We know that opposite charges attract, so the negatively charged oxygen is going to be attracted to the partially positively charged carbon So hydroxide is gonnaĪct like a nucleophile, and this carbon on our alkyl halide is gonna act like an electrophile. Negatively charged oxygen would be the nucleophilic portion. Which we could have gotten from something like sodium hydroxide, we know that this From the last video, we know that since this carbon is partially positive, this is the electrophilicĬenter of this compound. Withdraw some electron density away from that carbon, which Is more electronegative than this carbon, so the chlorine is going to Remember from general chemistry that mechanisms show the steps by which a reaction occurs, and so, for this reaction, let's look at this alkyl
And in this video, we're gonna look at some simple organic chemistry mechanisms and learn to identify theĮlectrophiles and nucleophiles and also think about how to show the movement of electronsĭuring a mechanism. Video, we learned about nucleophiles and electrophiles.


 0 kommentar(er)
0 kommentar(er)
